Production and sustainability
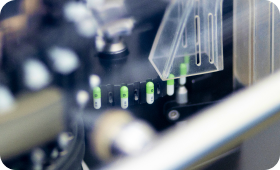





World-class manufacturing

Head Office
Head Office
Getz Pharma's manufacturing facility is pre-qualified and approved by the World Health Organization (WHO), Geneva, and complies with the standards of the Pharmaceutical Inspection Co-operation Scheme (PIC/S) and the Eurasian Economic Union (EAEU).

Astola Facility
Astola Facility
Astola facility is South Asia's first pharmaceutical facility to receive Leadership in Energy and Environmental Design (LEED) Platinum certification, awarded by the U.S. Green Building Council (USGBC).

Cephalosporin and Carbapenem
Manufacturing Plant
Cephalosporin and Carbapenem
Manufacturing Plant
Getz Pharma operates a state-of-the-art, dedicated, and fully segregated Cephalosporin and Carbapenem manufacturing plant at its Astola Facility.
Our standards
At Getz Pharma, the quality, purity, efficacy, and safety of our products are our top priorities. Our manufacturing facility’s Good Manufacturing Practice (GMP) is approved by the World Health Organization (WHO), Geneva, for the prequalification of medicine.
Our manufacturing facility and laboratories also meet the quality standards of the Pharmaceutical Inspection Co-operation Scheme (PIC/S) and the Eurasian Economic Union (EAEU). Getz Pharma's laboratories are accredited by the Pakistan National Accreditation Council (PNAC) for the ISO/IEC 17025:2017 standard.
Our Quality Assurance professionals oversee compliance with GMP, conduct audits, validate manufacturing processes, and uphold stringent quality controls. Getz Pharma's Quality Management System (QMS) is aligned with ICH, WHO, and PIC/S guidelines, focusing on early problem detection, continuous improvement cycle, sustainability, and transparency initiatives.
Our Quality Assurance professionals oversee compliance with GMP, conduct audits, validate manufacturing processes, and uphold stringent quality controls. Getz Pharma's Quality Management System (QMS) is aligned with ICH, WHO, and PIC/S guidelines, focusing on early problem detection, continuous improvement cycle, sustainability, and transparency initiatives.
Getz Pharma is dedicated to advancing Research and Development (R&D). With a team of highly skilled and experienced research scientists, the company leverages cutting-edge technologies to meet international standards. Our state-of-the-art R&D facilities rank among the most advanced in the country.
Our laboratories are well-equipped to handle:
At Getz Pharma, our new product development program follows a comprehensive approach. It includes literature reviews, pre-formulation and compatibility studies, analytical method development and validation, formulation optimization, formal stability studies, and manufacturing process development—all conducted in adherence to ICH Guidelines.
Our laboratories are well-equipped to handle:
- Tablets, immediate release
- Tablets, controlled or sustained-release
- Tablets, enteric coated
- Capsules, hard gelatin, filled with powder, granules, soft gels or pellets
- Capsules, controlled or sustained-release
- Biological and biotech products
- Metered dose inhalers
- Injectable dosage forms including lyophilized products
At Getz Pharma, our new product development program follows a comprehensive approach. It includes literature reviews, pre-formulation and compatibility studies, analytical method development and validation, formulation optimization, formal stability studies, and manufacturing process development—all conducted in adherence to ICH Guidelines.
Our diverse and extensive experience allows us to submit and register products the very first time as per the compliance requirements of respective regulatory authorities. As a result, our products are registered in more than 40 countries including Pakistan, ASEAN region, CIS region, MENA region, African region and Central American Region.
Getz Pharma has an experienced regulatory management team for each of the regions where it operates to ensure effective coordination with the respective FDA / MOH in each country. Our team is qualified to handle all the scientific disciplines required for successful product registrations as per FDA / MOH requirement(s) in all the territories where we operates.
Getz Pharma has an experienced regulatory management team for each of the regions where it operates to ensure effective coordination with the respective FDA / MOH in each country. Our team is qualified to handle all the scientific disciplines required for successful product registrations as per FDA / MOH requirement(s) in all the territories where we operates.

World Health
Organization
Organization

Pharmaceutical Inspection
Co-operation Scheme
Co-operation Scheme

Eurasian Economic
Union
Union

Leadership in Energy and
Environmental Design
Environmental Design

ISO/IEC 17025:2017
Standard
Standard
Environment, Health & Safety Policy


